Diagnosing Biofilm Infections
Biofilms lead to chronic inflammation and pain
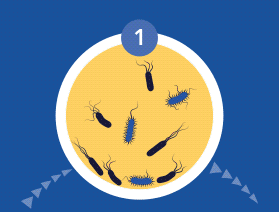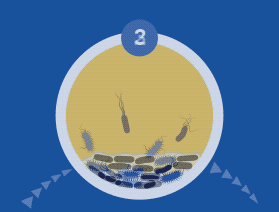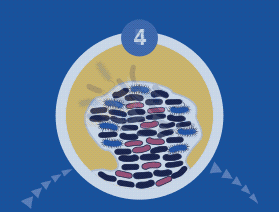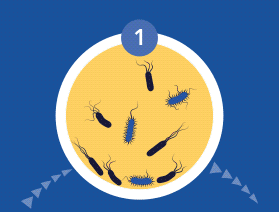
The CDC and NIH have estimated that biofilm infections now constitute 65-80% of bacterial infections treated by physicians in the developed world. Once in the prostatic ducts and acini, bacteria multiply and induce an acute inflammatory reaction. If untreated at this planktonic state, bacteria can then form biofilms that adhere to the epithelium of the ductal system. Here they produce exopolysaccharide slime or glycocalyx protective envelopes, which leads to persistent immunological stimulation and subsequent chronic inflammation and pain.
Clinical importance
Biofilm infections exhibit recalcitrance to antimicrobial compounds, and persistence in spite of sustained host defenses
Klebsiella pneumoniae has been shown to develop intracellular biofilms linked to the expression of type 1 pili and intracellular uropathogenic E. coli (UPEC) that exhibit the same phenotype in epithelial cells. Intracellular UPEC undergo a complex developmental process into biofilm aggregates within the epithelial cell cytoplasm.
A large percentage of E. coli strains collected from patients with prostatitis exhibit the ability to develop biofilms, which may explain the difficulty in treating of infections. Biofilm development may be crucial for UPEC persistence in the vagina and/or bladder, being related to persistence and recurrence.
During the colonization process, microbial cells communicate via quorum sensing (QS) using signal molecules such as acyl-homoserine lactones (AHL), in the case of Gram-negative species, and peptides in the case of Gram-positive bacteria. Once the bacterial cells firmly adhere to the implant or tissue’s surface, they aggregate through cell-to-cell interaction, producing a biopolymer extracellular matrix which provides both adhesion ability and scaffolding for the biofilm’s three-dimensional architecture.
Once the biofilm is formed, the bacterial species within it can retard reflexive treatment protocols. Proteus mirabilis produces the enzyme urease, which hydrolyzes urea to ammonia and carbon dioxide. The release of ammonia raises the urinary pH, which favors the precipitation of urinary salts forming kidney or bladder stones, which frequently serve as a nidus for recurrent P. mirabilis infection.
P. mirabilis also produces two toxins: hemolysin (HpmA), which destabilizes the host cell by inserting itself into the cell membrane, and Proteus toxic agglutinin (Pta), which produces holes in the host cell membrane, causing leakage of the cytosol, osmotic stress, and the depolymerization of actin filaments. Pta also induces bacterial cell-to-cell interaction via auto aggregation.
Enterococci also produce several adhesion factors involved in catheter-associated biofilm development, including the collagen adhesin Ace, the enterococcal surface protein (Esp), the enterococcal polysaccharide antigen (Epa), and the endocarditis- and biofilm-associated pili (Ebp). E. faecalis attaches to fibrinogen-coated catheters and uses it for growth, enhancing biofilm development on the catheter.
The environmental conditions created on the catheter surface make it an ideal site for bacterial attachment and formation of biofilm structures. In this type of medical device, urease-producing microorganisms may cause encrustation, formation of infected bladder calculi, and urinary obstruction. The crystal layer protects bacteria from the antimicrobial effects of compounds used for coating or impregnating the catheters. In addition, biofilm formation may even result in the increased ability of uropathogens to cause acute prostatitis and persist in the prostatic secretory system, leading to recurrent UTIs characteristic to chronic bacterial prostatitis.
How do biofilm bacteria escape culture detection?
A biofilm-mediated adaptation produces trade-offs that could limit the ability of bacteria to transition between biofilm growth and the free-living state and produce bacterial populations that escape detection by culture-based sampling.
Biofilm growth increases the fitness of bacteria in harsh conditions. However, bacteria from clinical and environmental biofilms can exhibit impaired growth in culture, even when the species involved are readily culturable, and permissive conditions are used.
For example, a culture-impaired variant of Pseudomonas aeruginosa undergoes mutations that alter the outer-membrane lipopolysaccharide structure. Within biofilms, the lipopolysaccharide mutations markedly increase bacterial fitness. However, outside the protected biofilm environment, the mutations sensitize the variants to killing by a self-produced antimicrobial agent.
Biofilms and antimicrobial resistance
Bacteria in a biofilm behave differently and are phenotypically very different from planktonic free‐floating bacteria. Biofilm bacteria can survive antimicrobial agents at concentrations 1000–1500 times higher than the concentrations that kill planktonic cells of the same species.
Biofilms are more resistant to antibiotics than planktonic cells due to:
- The limitation of antibiotic diffusion through the matrix.
- The transmission of resistance genes within the community.
- The expression of efflux pumps and the inactivation of the antibiotics due to changes in metal ion concentrations and pH values.
- The physiological changes of microbial cells due to nutrient limitation environment (reduced metabolic and growth rates).
- The presence of metabolically inactive cells (persisters or dormant bacterial cells) as bacteria enter a spore-like, non-dividing state that is more tolerant to antimicrobials.
- The induction of a biofilm phenotype (expression of active mechanisms to combat the detrimental effects of antimicrobial agents).
- Bacteria within a biofilm activate many genes that alter the cell envelope and molecular targets and the susceptibility to antimicrobial agents (intrinsic resistance). Phenotypic changes brought on by a genetic switch, when 65–80 proteins change, may play a much more important role in the protection from antimicrobial agents than the external resistance provided by the exopolysaccharide slime.
As 65-80% of bacterial infections are biofilm-related, MicroGenDX’s qPCR+NGS greatly improves the reliability and accuracy of diagnostic information – especially as compared to standard culture. MicroGenDX has proven its clinical utility in detecting and effectively treating urological biofilm-related infections.
References
- Choong S, Whitfield H. Biofilms and their role in infections in urology. BJU Int. 2000;86(8):935‐941. doi:10.1046/j.1464-410x.2000.00949
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034‐1043. doi:10.1111/j.1462-5822.2009.01323.x
- Nickel JC, Costerton JW. Coagulase-negative staphylococcus in chronic prostatitis. J Urol. 1992;147(2):398‐401. doi:10.1016/s0022-5347(17)37247-6
- Cha JO, Yoo JI, Yoo JS, et al. Investigation of Biofilm Formation and its Association with the Molecular and Clinical Characteristics of Methicillin-resistant Staphylococcus aureus [published correction appears in Osong Public Health Res Perspect. 2014 Apr;5(2):116]. Osong Public Health Res Perspect. 2013;4(5):225-232. doi:10.1016/j.phrp.2013.09.001
- Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens. 2014;3(3):743-758. Published 2014 Sep 5. doi:10.3390/pathogens3030743
- Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front Microbiol. 2017;8:1566. Published 2017 Aug 15. doi:10.3389/fmicb.2017.01566
- Delcaru C, Alexandru I, Podgoreanu P, et al. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens. 2016;5(4):65. Published 2016 Nov 30. doi:10.3390/pathogens5040065
- Penterman J, Nguyen D, Anderson E, et al. Rapid evolution of culture-impaired bacteria during adaptation to biofilm growth. Cell Rep. 2014;6(2):293‐300. doi:10.1016/j.celrep.2013.12.019
- Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322‐332. doi:10.1016/j.ijantimicag.2009.12.011
- G.D. Ehrlich et al. Culture Negative Orthopedic Biofilm Infections, Springer Series on Biofilms 7. 2012. doi.org/10.1007/978-3-642-29554-6_1