Due to the advances in human medicine, global mortality due to infections has dramatically reduced over the last century. However, the fight against infections is far from over. Microbial infections continue to pose a significant public health concern worldwide.
In 2015, lower respiratory tract infections (LRIs) accounted for 2.74 million deaths globally, and 55.4% of LRI deaths were caused by Pneumococcal pneumonia. The same year saw 1.31 million deaths attributed to diarrheal diseases caused by pathogens such as Salmonella spp, Shigella spp, and Rotavirus. Most notably, the global incidence of tuberculosis rose to 10.2 million, contributing to a large proportion of the disease burden.
Infections can affect any organs and may arise due to the organism’s virulence factors, or the body’s responses toward the infection. Pathogens can cause direct damage to cells and tissues, but at times the body’s immune system may also contribute to disease manifestations when responding to infections.
Microbial infections may be acquired through various modes — contact, droplets, vectors (e.g., animals, insects) and vehicular through food and water. Infectious diseases could either be contagious from person to person or non-contagious.
Increasingly, there have been rising concerns over emerging infectious diseases (EIDs), which are newly recognized infections that have recently expanded in incidence rates, new infections caused by changed or evolved micro-organisms, or re-emerging infections due to the development of antimicrobial resistance.
Over the past five years, there have already been more than 12 emerging pathogens and diseases. EIDs are highly contagious and have potentially severe implications on global health, the economy and security.
The Rise of Emerging Infectious Diseases
 Although there still gaps in knowledge on the causes of EIDs, studies have pointed to zoonotic pathogens (animal origins) as causative agents to a majority of EIDs and that there are several biological and socio-environmental factors working in tandem to drive the spread of zoonotic pathogens and hence, the rise of EIDs.
Although there still gaps in knowledge on the causes of EIDs, studies have pointed to zoonotic pathogens (animal origins) as causative agents to a majority of EIDs and that there are several biological and socio-environmental factors working in tandem to drive the spread of zoonotic pathogens and hence, the rise of EIDs.
Because of the novelty of EIDs, they are unpredictable, difficult to diagnose, and therefore have the potential to be life-threatening. Experts believe that new and robust diagnostic methods that have broad-range capabilities are required to better survey and manage EIDs.
Antibiotic-resistance infections (ARIs), a form of EID, are of particular concern. ARIs are caused by microbes that have acquired the ability to resist and survive even in the presence of antibiotics that were previously effective at killing them. This results in infections that are increasingly difficult to treat.
According to the CDC, more than 2.8 million ARIs and more than 35,000 ARI-related deaths are reported in the United States annually. Several microbes are classified as “urgent threats” due to their ability to cause severe infections and diseases such as pneumonia, urinary tract infections, diarrhea, and gonorrhea. Additionally, ARIs affect surgeries, organ transplantations, cancer treatment, kidney dialysis and other chronic health conditions.
Despite making great strides in human medicine and public health policies, we are still in an ongoing battle against infections. Over-prescription and misuse of antibiotics are the underlying causes of antibiotic resistance. Improving antibiotic stewardship, as well as early detection and surveillance, are some of the strategies proposed by CDC to combat ARIs.
Current challenges
 Proper diagnosis of infections is crucial in guiding treatments and patient management. Today, many practitioners rely on conventional diagnostic approaches for microbial infections such as microbial cultures and microscopy because they are recognized as ‘gold standards’ of diagnostic tests.
Proper diagnosis of infections is crucial in guiding treatments and patient management. Today, many practitioners rely on conventional diagnostic approaches for microbial infections such as microbial cultures and microscopy because they are recognized as ‘gold standards’ of diagnostic tests.
Microscopy involves microbial identification based on staining properties and cell morphologies, while microbial cultures involve growing microbe samples in a nutrient-rich agar plate or broth.
There are several limitations to microbial cultures:
- Pathogens in lower concentrations may not grow fast enough to be detected in culture.
- Not all microbes grow under standard culture conditions, resulting in false negatives.
- Slow growing mycobacterium and fungal cultures can delay treatment
- Variability among Laboratories may impact comparability of laboratory results
Biofilms formed due to microbes’ aggregation and adhesion are difficult to detect via conventional culturing methods because microbes in biofilms cannot be easily grown in standard cultures.
With the advancements in diagnostic technologies, immunochromatography-based Rapid Diagnostic Tests (RDTs) were developed, improving the ease of detecting a range of infections such as LRIs, diarrheal and gastrointestinal infections. More advanced RDTs even allow multiplex detection (detection of more than one pathogen).
However, RDTs are often expensive and are still limited to the number of pathogens they can detect. RDTs also require additional confirmatory tests to supplement test results. Lastly, practices for implementing RDTs in healthcare facilities are not standardized.
Other infections are simply intrinsically challenging to diagnose via traditional methods due to biological reasons such as close similarities between microbial subspecies, e.g., Mycobacterium infections are often misdiagnosed due to difficulties in distinguishing between Mycobacterium subspecies.
The future of infection management
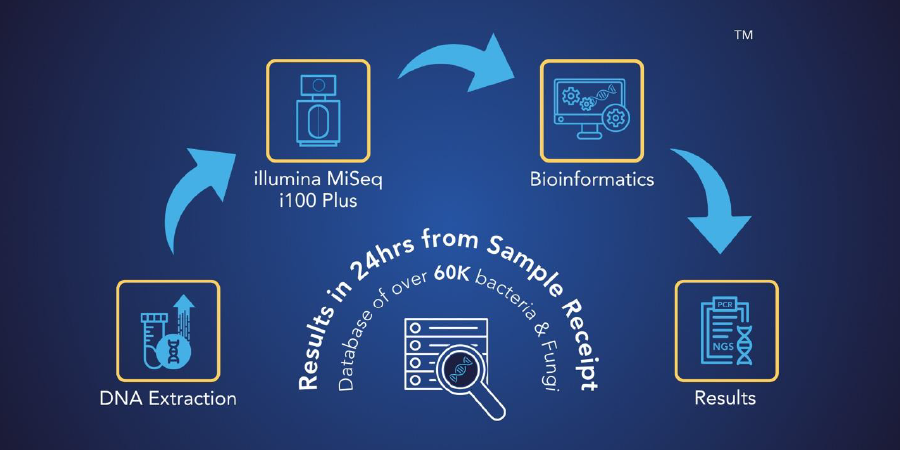
 MicroGenDX next-generation sequencing (NGS) has revolutionized the way infections are diagnosed. MicroGenDX can efficiently sequence the DNA of microbes present within days, thereby rapidly and accurately identifying microbes present in samples. NGS can even pick up microbes that are small in numbers.
MicroGenDX next-generation sequencing (NGS) has revolutionized the way infections are diagnosed. MicroGenDX can efficiently sequence the DNA of microbes present within days, thereby rapidly and accurately identifying microbes present in samples. NGS can even pick up microbes that are small in numbers.
With the persistence of infections and increasing incidence of EIDs, accurate diagnosis and, therefore, treatment of infections are vital in improving patient care quality. Dr. Jon Minter, an orthopedic surgeon in Atlanta, Georgia, has integrated MicroGenDX solutions as part of his clinical practice and infection management strategy.
He highly recommends MicroGenDX to his colleagues, citing reliable results, fewer negative results and in-depth information as his main reasons. Delivering appropriate treatments reduces misuse of antibiotics and improves antibiotic stewardship, which is especially crucial in light of ARIs threats. Improved diagnostic strategies will also help control the spread of pathogens.
Next-generation sequencing provides a comprehensive approach to diagnosing infectious diseases
 MicroGenDX, the leader in molecular diagnosis of infections, offers reliable and affordable diagnostic services that combine qPCR and NGS to facilitate the diagnosis of infections. MicroGenDX’s NGS services can differentiate among 20,000 bacterial and 30,000 fungal species, quantify overall bacterial burden, and identify dominant species — in just 3 to 5 days. MicroGenDX’s qPCR + NGS can detect the presence of antibiotic-resistant genes, allowing clinicians to prescribe the appropriate treatments to patients.
MicroGenDX, the leader in molecular diagnosis of infections, offers reliable and affordable diagnostic services that combine qPCR and NGS to facilitate the diagnosis of infections. MicroGenDX’s NGS services can differentiate among 20,000 bacterial and 30,000 fungal species, quantify overall bacterial burden, and identify dominant species — in just 3 to 5 days. MicroGenDX’s qPCR + NGS can detect the presence of antibiotic-resistant genes, allowing clinicians to prescribe the appropriate treatments to patients.
MicroGenDX’s qPCR + NGS solution can detect microbes in wounds, improving the management of chronic non-healing wounds. Overall, this approach effectively minimizes false negatives while comprehensively understanding both infection and disease.