Diagnosing and Treating Biofilm Infections
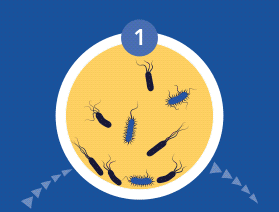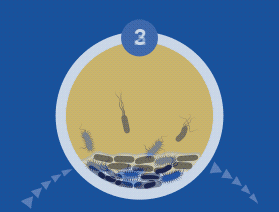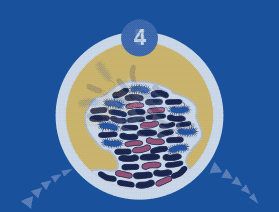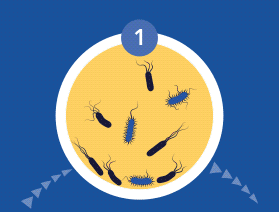
Planktonic vs. biofilm phenotypes
All microorganisms can exist in both the planktonic (fee-living) and biofilm phenotypic states, but tend to gravitate towards a biofilm phenotype due to their preference for attaching to each other and to surfaces. Biofilm formation is now recognized as causing or exacerbating numerous chronic infections, including chronic rhinosinusitis and chronic otitis media (OM). Biofilm formation has been linked to Haemophilus influenzae and Streptococcus pneumoniae in chronic otitis media, and to Staphylococcus aureus in chronic rhinosinusitis.
Culture can’t adequately detect biofilm bacteria, but MicroGenDX NGS can
A biofilm can have multiple gradients of anoxic and acidic zones in the interior of biofilm clusters, requiring differentiated media, growth conditions, and varying lengths of time to cultivate — all of which are not supported by standard culture.
A biofilm-mediated adaptation also produces trade-offs that could limit the ability of bacteria to transition between biofilm growth and the free-living state, and thereby generate a specimen that may escape detection by culture-based sampling.
Next-generation sequencing (NGS) can identify the multiple bacteria and fungi that make up a biofilm, along with the clinically relevant distributions of each in the sample, which is a critical starting point in understanding a biofilm’s involvement in a given infection.
Biofilms are more resistant to antimicrobials than planktonic cells
Bacteria within a biofilm activate many genes that alter the cell envelope and molecular targets, as well as altering the susceptibility to antimicrobial agents (intrinsic resistance). Current opinion is that phenotypic changes brought on by a genetic switch, where 65–80 proteins change, play a hugely important role in protection from antimicrobial agents.
In addition, biofilms can achieve greater antimicrobial resistance through one or more of the following conditions:
- Transmission of resistance genes within the bacterial community
- Reduced metabolic and growth rates from a nutrient-limited environment
- The presence of metabolically inactive cells known as persisters, where bacteria enter a spore-like, non-dividing state that is more tolerant to antimicrobials
- The induction of a biofilm phenotype (i.e the expression of active mechanisms to combat the detrimental effects of antimicrobial agents)
References
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034‐1043. doi:10.1111/j.1462-5822.2009.01323.x
- Vuotto C, Longo F, et al. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens. 2014;3(3):743-758. Published 2014 Sep 5. doi:10.3390/pathogens3030743
- Penterman J, Nguyen D, et al. Rapid evolution of culture-impaired bacteria during adaptation to biofilm growth. Cell Rep. 2014;6(2):293‐300. doi:10.1016/j.celrep.2013.12.019
- Høiby N, Bjarnsholt T, et al. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322‐332. doi:10.1016/j.ijantimicag.2009.12.011
- Ehrlich G D et al. Culture Negative Orthopedic Biofilm Infections, Springer Series on Biofilms 7. 2012. doi.org/10.1007/978-3-642-29554-6_1
- Jamal M, Ahmad W, Andleeb S, et al. (2018). Bacterial biofilm and associated infections. J. Chin. Med. Assoc.81, 7–11. doi: 10.1016/j.jcma.2017.07.012