Molecular testing is personalized healthcare at its most advanced. Still, because molecular diagnostics is a relatively young field fueled by new and emerging technologies, it’s essential for physicians to capture detailed data to establish medical necessity, determine payer responsibility and process reimbursement.
Comprehensive test requisition forms and precise medical coding to support medical necessity are especially important for proprietary lab analyses (PLAs), such as those offered by MicroGenDX, and out-of-network lab tests, an umbrella under which many DNA-based diagnostics fall. As molecular tests may leave some patients facing out-of-pocket expenses, it’s also paramount for physicians to discuss this potential based on an individual’s health coverage or lack thereof.
The State of Medical Coding
In the drive to meet federal interoperability and patient-access guidelines1 and adapt to evolving electronic medical record (EMR) standards, accurate and consistent medical coding is perhaps more important than ever. Thanks to the persistent COVID-19 pandemic and ongoing healthcare labor shortage, it is also more challenging than ever to achieve.
New International Classification of Diseases (ICD) codes associated with COVID diagnostics and treatment led to confusion and inconsistencies amid the pandemic’s peak.1 This was followed by an increase in claims denials,2 often stemming from inaccurate or incomplete medical coding.
These problems have been magnified by the labor crisis, as many physicians and remaining staff take on additional coding input responsibilities. To cope, some providers have taken a minimalistic approach to coding when it comes to certain medical necessity, diagnostic and patient-history details.
Unfortunately, this method does not streamline data and workflow. Instead, it results in information gaps that others must fill in later, contributing to patient confusion about who is paying for specific services. Inadequate coding can disrupt the billing and reimbursement cycles — and diminish the patient experience.
Molecular Testing and Medical Necessity
For molecular diagnostic testing, claims will sometimes be paid based solely on the diagnosis code provided on the test requisition form. But additional information is often needed to establish medical necessity, and it’s crucial for physicians to complete the requisition form with the most specific diagnosis code available, as opposed to just a broad category code.
To support medical necessity, facilitate the requested tests and ascertain reimbursement steps, pertinent data is needed regarding patient medical history, patient assessment, why a particular test was requested, what the physician hopes to gain from the test results, and potential treatment plans. The American Academy of Professional Coders (AAPC) recommends these general ICD-10 coding best practices for verifying medical necessity3:
- List the principal diagnoses, condition, problem or other reason for the medical service.
- Assign the code with the highest level of specificity.
- For outpatient services, do not use a “rule-out” statement or a suspected but unconfirmed diagnosis; if a definitive diagnosis is not yet determined, code the symptoms instead.
- Be explicit when describing the patient’s condition, and distinguish between acute and chronic conditions as appropriate.
- Identify chronic complaints or secondary diagnoses if treatment is provided or if they impact patient-care management.
Capturing more details than the basic codes is particularly consequential when requesting specialized testing like molecular diagnostics. The microbial DNA diagnostics provided by MicroGenDX, for example, rely on new technology and proprietary laboratory analysis. As a result, there are not currently many payers whose coverage encompasses these types of tests; in fact, payers often attach preauthorization requirements to specialized diagnostics providers.
The latter point is why MicroGenDX pursued its own Medicare-accepted current procedural terminology (CPT) code for its quantitative polymerase chain reaction (qPCR) and next-generation sequencing (NGS) diagnostics. This CPT code (0112U) and other individualized PLA designations allow laboratories and test manufacturers to differentiate their diagnostics, offer payers details about what’s incorporated in the tests, and provide a foundation for broader coverage over time as more payers accept the necessity and validity of these tests.
In the meantime, however, physicians must remember to use these codes and bear in mind that some specialized diagnostics, like microbial DNA testing, are not covered by most private insurance plans. Even if medical necessity is established, partial or full payment for these tests may ultimately fall on patients.
Talk With Patients About Potential Out-of-Pocket Costs
In response to rising consumer complaints about surprise medical billing, the Centers for Medicare & Medicaid Services (CMS) instituted new rules that healthcare providers and payers must follow to prevent patients from receiving unexpected balance-due bills for out-of-network services.
These rules include explaining to insured patients that out-of-network services could be more expensive and giving uninsured patients a good-faith estimate of the recommended services’ costs before providing them. These regulations are intended to reduce surprise medical bills but collecting payer data and having a cost conversation about specialized diagnostics with patients upfront also sets the stage for a more efficient claims process. This conversation also improves the patient experience by furnishing realistic expectations about potential out-of-pocket costs.
MicroGenDX diagnostics are covered by Medicare Part B, although some Medicare Advantage members may require prior authorization and have a copay. MicroGenDX has further contracted with several state Medicaid plans as well as Tricare and select commercial payers.
MicroGenDX also offers discounted cash rates for private-pay patients. Additional information is available on our Insurance and Payment page.
About MicroGenDX: Get More Answers
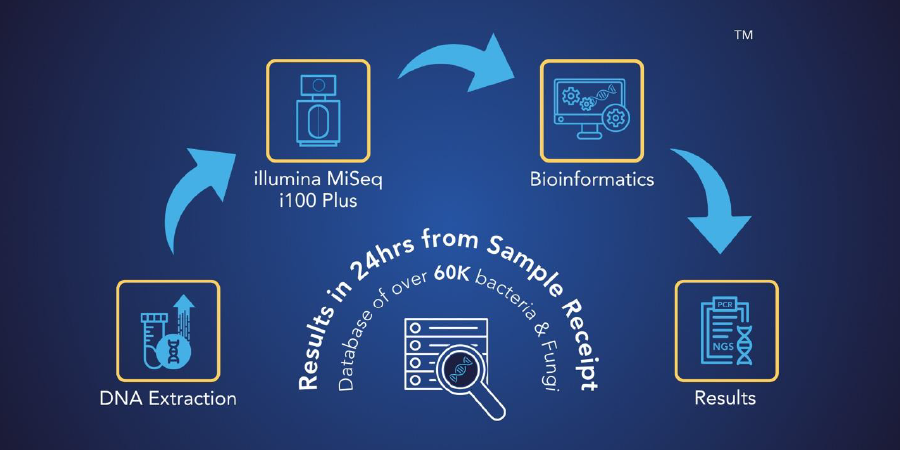
MicroGenDX is an innovative, CAP-accredited, CLIA-licensed molecular diagnostic laboratory that offers two-part DNA testing services that utilize our proprietary PCR and NGS technology.
Our process uses our proprietary bioinformatics system and curated database that provides precise detection of infectious diseases at high levels of sensitivity and specificity. Our mission is to improve clinical outcomes by offering physicians and patients the most informative and impactful microbial diagnostics that science can provide.
Resources
- LaPointe, Jacqueline. “Providers Confused by Coding, Claim Requirements,” RevCycle Intelligence. Aug. 31, 2020. Retrieved May 8, 2022. https://revcycleintelligence.com/news/providers-confused-by-covid-19-coding-claim-requirements
- Lagasse, Jeff. “More than 30% of Hospitals are Near ‘Danger Zone’ of Denial Rates,” Healthcare Finance News. June 18, 2021. Retrieved May 8, 2022. https://www.healthcarefinancenews.com/news/more-30-hospitals-are-near-danger-zone-denial-rates
- Verhovshek, John. “Medical Necessity: Why it Matters, Ways to Demonstrate It,” American Academy of Professional Coders Knowledge Center. April 5, 2019. Retrieved May 8, 2022. https://www.aapc.com/blog/46500-medical-necessity-why-it-matters-ways-to-demonstrate-it/