Chronic infection and chronic non-healing wounds are devastating to the patient, a challenge to the healthcare provider, and costly to the healthcare system. Quite often, the culprit is biofilm.
Biofilm is a thin coating consisting of bacteria embedded in a moist, adhesive matrix covering living tissues and indwelling medical devices like catheters, stents, pacemakers, and prosthetics. This matrix protects the organism, making it resistant to phagocytosis by white blood cells and killing by antibiotics. Biofilms are a huge medical problem because they make bacterial infections extremely difficult to diagnose and treat.
Biofilm and Antibiotic Tolerance/Resistance
According to a study in the Journal of Antimicrobial Resistance & Infection Control, approximately 80% of chronic and recurrent microbial infections in the human body are due to bacterial biofilm. Microbial cells within biofilms have shown 10 to 1,000 times more antibiotic resistance than the planktonic/free-floating cells.
Healthcare providers must often use incomplete or imperfect information to diagnose an infection and thus prescribe an antimicrobial “just in case” or prescribe a broad-spectrum antimicrobial when a specific antibiotic might be better. According to the National Institute of Allergy and Infectious Diseases, these situations contribute to and accelerate antimicrobial resistance.
Research shows there are three primary ways that antibiotic resistance occurs:
- Selective pressure – In the presence of an antimicrobial, microbes either survive if they carry resistance genes or die. The survivors will replicate, and their progeny will quickly become the dominant type throughout the microbial population.
- Mutation – Most microbes reproduce by dividing every few hours, allowing them to evolve rapidly and adapt quickly to new environmental conditions. During replication, mutations arise, and some of these mutations may help an individual microbe survive exposure to an antimicrobial.
- Gene Transfer – Microbes also may get genes from each other, including genes that make the microbe drug-resistant. Bacteria multiply by the billions. Bacteria that have drug-resistant DNA may transfer a copy of these genes to other bacteria. Nonresistant bacteria receive the new DNA and become resistant to drugs. In the presence of drugs, only drug-resistant bacteria survive, multiply and thrive. This mode of developing resistance is most concerning is the presence of biofilm as bacterial colonization facilitates gene transfer.
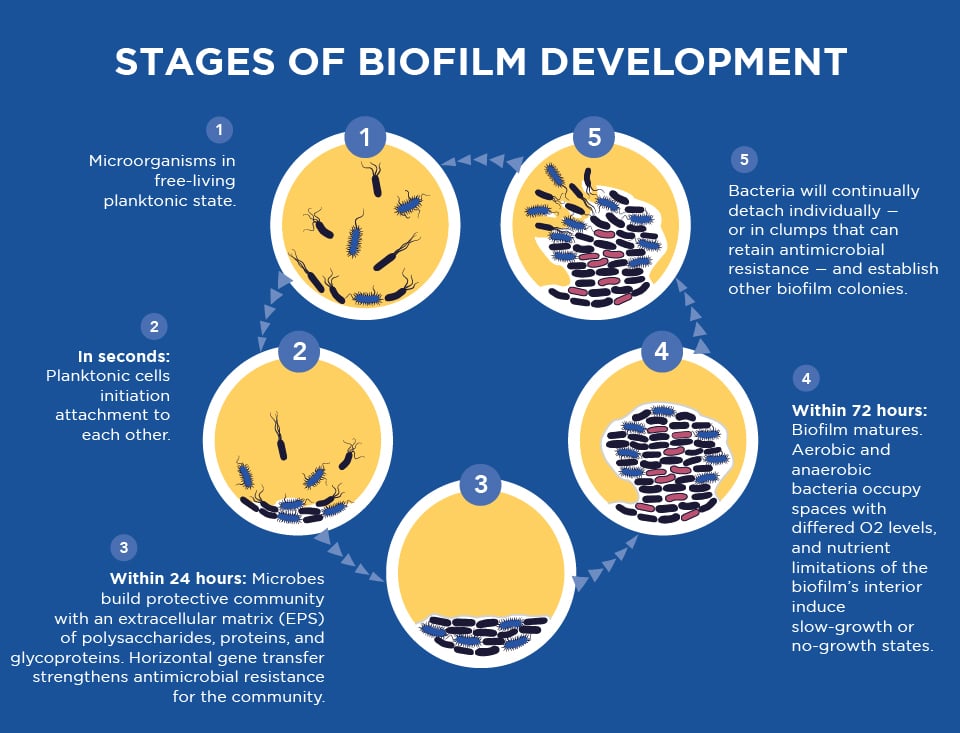
The Failure of Traditional Testing
Traditional culture technology is often inadequate in the presence of biofilm. The reason for this lies in the fact that bacteria in a biofilm behave differently and are phenotypically different from planktonic/free‐floating bacteria. This is evidenced by the fact that biofilm bacteria exhibit impaired growth in culture, which can result in a false negative result even in the presence of obvious infection. Biofilm bacteria are also more resistant to antibiotics than when in their free-floating form.
Biofilms can achieve greater antimicrobial resistance through one or more of the following conditions:
- Transmission of resistance genes within the bacterial community
- Reduced metabolic and growth rates from a nutrient-limited environment
- The presence of metabolically inactive cells known as persisters, where bacteria enter a spore-like, non-dividing state that is more tolerant to antimicrobials
- The induction of a biofilm phenotype (i.e the expression of active mechanisms to combat the detrimental effects of antimicrobial agents)
MicroGenDX’s Next-Generation Sequencing
 By extracting and analyzing the DNA of the involved organisms, MicroGenDX’s Next-Generation Sequencing can identify the multiple bacteria and fungi that make up a biofilm, along with the distributions of each within a sample. This is critical in understanding a biofilm’s involvement in an infection or chronic wound and initiating targeted, effective treatment sooner than is possible with traditional culture testing.
By extracting and analyzing the DNA of the involved organisms, MicroGenDX’s Next-Generation Sequencing can identify the multiple bacteria and fungi that make up a biofilm, along with the distributions of each within a sample. This is critical in understanding a biofilm’s involvement in an infection or chronic wound and initiating targeted, effective treatment sooner than is possible with traditional culture testing.
Why more medical professionals are choosing MicroGenDX’s Next-Generation Sequencing:
- Improved patient outcomes
- 75% less expensive than the majority of labs offering this technology
- Fast results (3-5 days / 3.5-day average)
- Detection of bacteria in the presence of antibiotics
- Specificity of 99.9% of microbes within an infection site
- Simplicity of sample collection
According to their DNA, the ability to identify microbes has changed the way many physicians view microbial infections and their treatment. Providers who use it say MicroGenDX’s Next-Generation Sequencing improves patient outcomes and saves lives.
Dr. Michael Waters, a urologist at the Texas Center for Urology, has seen several patients in whom he’s confident there was an infection. Yet, an average of 30% to 35% of cultures returned no growth. The first patient he used Pathogenius/MicrogenDX on was a patient with chronic hematospermia. The patient had seen multiple physicians for several years, with multiple urinalysis and cultures returning negative results.
“An MRI was performed to the pelvis demonstrating some mild symptoms of vesiculitis. The ultrasound was negative for abscess. Calcifications, his PSA was within normal limits. When we got the semen analysis back, the Pathogenius / MicroGen DX showed a biofilm in the effect of anaerobic bacteria,” Dr. Waters said. “This made a significant difference in the patient’s care and his outcome. I’m not sure what would have happened if I hadn’t come across the pathogenius/ MicroGenDX technique and DNA sequencing.
“When I was able to provide him with targeted therapy and placed him on antibiotics he’d never been on before and get him turned around as he did. The last time I saw the patient he just hugged me — it was a great relief for him.”
Dr. Eric L. Johnson, medical director of Bozeman Deaconess Wound and Hyperbaric Center, believes clinicians not using Next-Generation Sequencing are behind the times. Ignoring biofilm, which is well identified in the wound care world, will only hinder progress.
“In terms of cost versus time versus pain and suffering … it’s well worth it. In our cost-value analysis, there’s not been at all a loss. It’s been a very positive thing to our patients in our system,” Dr. Johnson said. “Moving from culture to DNA Next-Gen sequencing is just a natural evolution in terms of resolving your biofilm problem and getting patients healed quicker.”
By identifying the multiple bacteria and fungi that make up a biofilm, along with the clinically relevant distributions of each in the sample, MicroGenDX’s next-generation sequencing is a critical starting point in understanding a biofilm’s involvement in a given infection.
To learn more, visit microgendx.com/biofilm-research.