Periprosthetic joint infections (PJI) remain one of the most serious and costly complications associated with total joint arthroplasty.
Although they occur in 1–2% of cases, the consequences are severe—ranging from multiple revision surgeries and prolonged hospital stays to reduced patient mobility and increased mortality risk. Beyond clinical outcomes, the financial burden is substantial, with average cost of $100,000 per episode and lifetime costs reaching roughly $391,000 per patient.
A particularly complex clinical challenge arises when PJIs are culture-negative, which occurs in an estimated 15–30% of cases. When standard microbiological cultures fail to identify a culprit pathogen, orthopedic surgeons and infectious disease specialists must rely on empirical therapies. These broad-spectrum regimens may be either insufficient or too aggressive, increasing the risk of treatment failure, antimicrobial resistance development, and extended recovery timelines.
As revision procedures increase in volume and arthroplasty cases increase worldwide by 2030, orthopedists seek to improve treatment durability.
In this clinical landscape, Next-Generation DNA Sequencing (NGS) is emerging as a powerful diagnostic aid that delivers actionable insights when culture falls short. By analyzing DNA directly from synovial fluid or periprosthetic tissue, NGS enables tailored therapies.
This blog explores how recent studies and patient cases, including new research led by Dr. Alisina Shahi of UT Health Houston and a large multicenter analysis published in key orthopedic journals, demonstrate NGS’s impact on management of culture-negative PJIs.
Why Laboratory Culture Fails to Diagnose PJI
 Despite being considered the gold standard for infection diagnosis, culture faces significant limits in PJI detection. Many PJI pathogens exist in a sessile, biofilm-protected state on implant surfaces, where their reduced metabolic activity makes them difficult to detect through standard culture conditions.
Despite being considered the gold standard for infection diagnosis, culture faces significant limits in PJI detection. Many PJI pathogens exist in a sessile, biofilm-protected state on implant surfaces, where their reduced metabolic activity makes them difficult to detect through standard culture conditions.
Further complicating diagnosis is the frequent involvement of low-virulence organisms such as Cutibacterium acnes, Finegoldia magna, and coagulase-negative staphylococci. These pathogens may grow slowly, require specific anaerobic environments, or be overshadowed in polymicrobial infections.
Additionally, many patients receive antibiotics before sample collection, which suppresses bacterial growth and reduces likelihood of a positive culture. Antibiotic use also reduces sensitivity of biomarkers used in the ICM criteria for PJI.
Moreover, mounting evidence (such as the studies below) suggests PJIs are often polymicrobial. Culture media can be biased to grow certain organisms, overrepresenting their place in an infection’s microbial community, while Viable but Nonculturable (VNBC) bacteria escape undetected.
These forms of diagnostic uncertainty, particularly in culture-negative PJIs, leave clinicians without a confirmed etiology to guide therapy. This often forces the use of broad-spectrum empiric antibiotics, which can increase toxicity risk, contribute to antimicrobial resistance, and complicate decisions regarding one-stage versus two-stage revision strategies.
To address these diagnostic gaps, more sensitive tools are required, particularly when conventional methods fail. This need has catalyzed interest in NGS as a diagnostic aid to detect microbial DNA directly from synovial fluid and/or periprosthetic tissue.
Introducing OrthoKEY: Built to Help Manage Orthopedic Infections
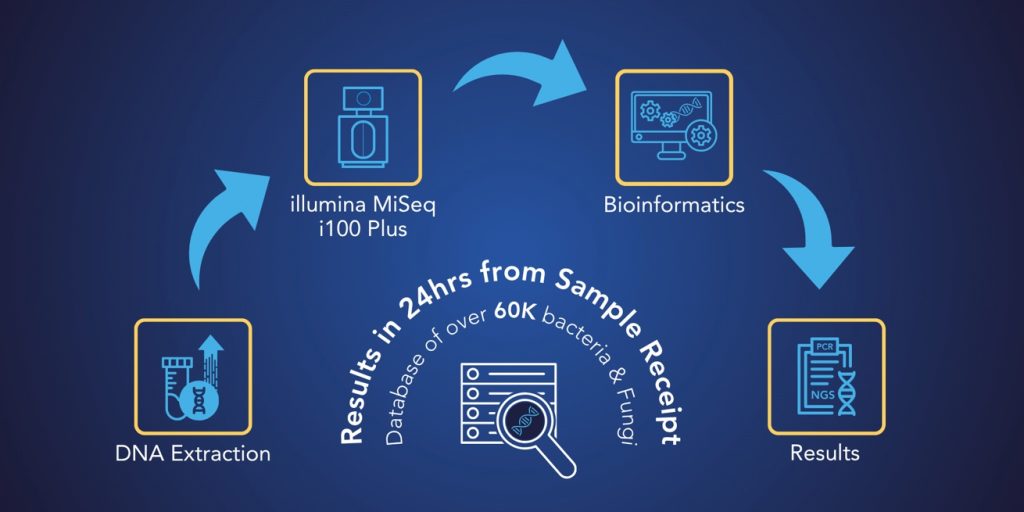 OrthoKEY is MicroGenDX’s qPCR + NGS test for PJI, offered in two versions: OrthoKEY Clinic for in-office aspiration samples and OrthoKEY OR for surgical tissue and implant samples.
OrthoKEY is MicroGenDX’s qPCR + NGS test for PJI, offered in two versions: OrthoKEY Clinic for in-office aspiration samples and OrthoKEY OR for surgical tissue and implant samples.
It combines sequencing, resistance profiling, and synovial biomarkers into a single test to support quicker personalized therapy, antimicrobial stewardship, and surgical decision-making.
It integrates:
- NGS for broad, unbiased detection of 60,000+ pathogens, including low-virulence, anaerobic, and biofilm-associated microbes – including atypical and mutated strains.
- qPCR amplification for rapid confirmation of key pathogens and detection of 17 types of antimicrobial resistance (AMR) genes.
- Synovial biomarker insights by testing for synovial CRP, WBC count, and PMN% to support correlation with MSIS / ICM diagnostic criteria.
- This also puts the microbes NGS detects in context of a potential PJI.
- Next-day reporting after sample receipt, enabling treatment guidance during early phases of care.
For support on complex cases, MicroGenDX provides free peer-to-peer support, connecting surgeons and ID specialists with experts familiar with MicroGenDX testing. This consultation service has helped providers interpret results and move confidently toward precision treatment.
OrthoKEY’s clinical value is best illustrated by the below peer-reviewed studies and patient cases.
Study Highlight #1: A Breakthrough in Culture-Negative PJI Detection
A recent study led by UTHealth Houston’s Chief of Arthroplasty, Dr. Kenneth Mathis and Adult Reconstruction Fellow, Dr. Alisina Shahi, titled What Culture Can’t See: NGS Identifies Pathogens in 80% of Culture-Negative PJIs, provides strong clinical evidence supporting NGS as a frontline diagnostic option in ambiguous PJI cases. Presented by Dr. Mathis, the study earned a coveted podium presentation slot against 2,200 other applications at 2025’s American Association of Hip and Knee Surgeons (AAHKS) Annual Meeting.
This study, conducted from 2020 to 2024 using MicroGenDX sequencing workflows, evaluated 131 patients with culture-negative PJIs defined by Musculoskeletal Infection Society (MSIS) criteria.
Tissue and synovial samples were submitted for NGS testing, and outcomes were assessed based on organism identification, effect on antibiotic therapy, time to results, and one-year reinfection/reoperation rates. The findings were clinically significant:
- NGS identified at least one organism in 81.8% of culture-negative PJIs.
- Antimicrobial therapy was modified in 68% of NGS-positive cases, enabling targeted treatment over broad empiric regimens.
- Median turnaround time for NGS was 3.4 days, compared to 5.7 days for traditional culture methods.
- Our testing turnaround is now even faster with results as soon as next-day after sample receipt.
- 1-year reinfection and reoperation rates were comparable to those in a matched cohort of culture-positive PJIs, validating the efficacy of NGS-guided care.
- NGS could help diagnose PJI without effect from premature antibiotic use.
By contrast, cell-free DNA (cfDNA) assays added new diagnostic information in about 10% of non-PJI hospital cases. MicroGenDX’s targeted NGS workflow identified pathogens in 80% of culture-negative PJIs and influenced antimicrobial therapy in 68% of those cases – showcasing the improved clinical utility of site-directed sequencing.
Study Highlight #2: Multicenter JBJS Study Confirms NGS Superiority in Large-Scale PJI Cohort
Further validation of NGS as a diagnostic tool for culture-negative PJIs emerged from a landmark multicenter study published in The Journal of Bone & Joint Surgery (JBJS)—the largest PJI-focused NGS analysis to date.
This study evaluated 301 patients who met the ICM criteria for PJI across more than a dozen leading orthopedic institutions in the United States, including the Rothman Institute, Cleveland Clinic, Rush University Medical Center, and Hospital for Special Surgery.
Of the total cohort, 85 cases (28.2%) were culture-negative, highlighting the frequency and clinical weight of diagnostic uncertainty in PJI care.
When NGS was applied:
- Pathogens were identified in 65.9% of culture-negative cases.
- A striking 91–93% of these NGS-positive cases were polymicrobial, as culture missed complex infections.
- 176 different species were found across all culture-negative cases, including 18 species that met a ≥5% incidence threshold, confirming many PJIs involve low-virulence or biofilm-associated microbes.
This study reinforced two critical observations:
1. Standard cultures frequently miss active infections.
2. PJIs are often polymicrobial rather than monomicrobial in nature.
The ability of NGS to uncover mixed microbial communities gives surgeons and infectious disease specialists a clearer understanding of infection severity, influencing choices on one-stage vs. two-stage revision strategies.
The breadth of institutional participation and scale of the data set make this multicenter analysis a pivotal reference point, confirming NGS delivers a more complete representation of infection microbiology than culture alone.
Study Highlight #3: Fixed PCR Panels vs. NGS in Real-world PJI
A Mayo Clinic study compared the BioFire Joint Infection (JI) multiplex PCR panel to targeted 16S NGS for diagnosing PJIs in patients with knee arthroplasty failure.
Using 60 synovial fluid samples classified under IDSA diagnostic criteria, researchers found the JI panel detected 56% of cases, while NGS detected 93%, with both showing similar specificity.
The JI panel was unable to detect off-panel microbes, mainly Staphylococcus epidermidis
– a leading PJI cause that accounted for 29% of culture-positive cases in the study.
Key Findings:
- Overall sensitivity: JI panel at 56% vs NGS at 93%
- On-panel organisms only: JI at 91% vs NGS at 96%
Fixed-target PCR panels are limited by the pathogens included in their design and lack the broad detective power needed for accurate diagnosis in culture-negative, polymicrobial, or low-virulence infections. In contrast, NGS delivers unbiased, high-sensitivity detection clinicians can rely on.
Case Spotlight #1: Rapid NGS Result Enables Targeted Therapy in Culture-Negative PJI
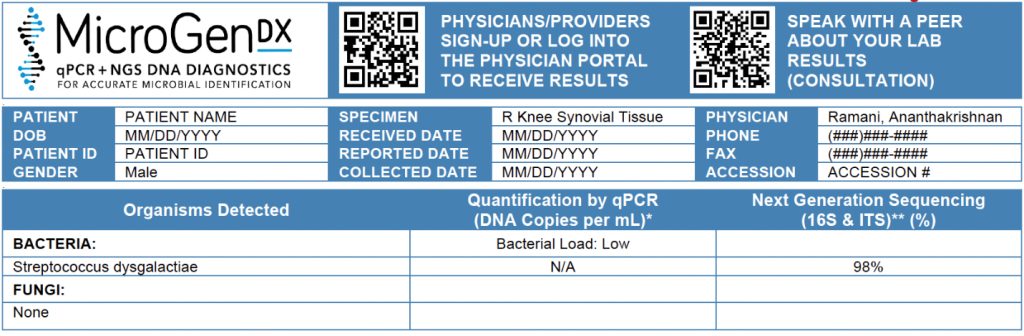 At Albany Medical Center, a 77-year-old male presented with a PJI of the right knee following total knee arthroplasty. After failing outpatient treatment with gentamicin and polymyxin irrigation, he underwent explantation with placement of an antibiotic spacer infused with vancomycin and tobramycin.
At Albany Medical Center, a 77-year-old male presented with a PJI of the right knee following total knee arthroplasty. After failing outpatient treatment with gentamicin and polymyxin irrigation, he underwent explantation with placement of an antibiotic spacer infused with vancomycin and tobramycin.
Synovial fluid and tissue cultures were obtained before new antibiotics were started. After 48 hours, no growth was observed. With the patient remaining culture-negative but still requiring continued antimicrobial therapy, the physician initiated empiric IV vancomycin and cefazolin.
To avoid prolonging therapy without confirmed pathogen(s), tissue samples were sent to MicroGenDX. NGS testing identified Streptococcus dysgalactiae, prompting a rapid and targeted change in management. Empiric therapy was discontinued, and the patient was discharged on IV ceftriaxone once daily.
The treating physician, Dr. A. Ramani, noted that next-day NGS results spared the patient from being discharged on multiple antibiotics requiring several daily doses. This reduced toxicity risk, improved adherence, and supported a more streamlined care plan while reinforcing antibiotic stewardship.
This case exemplifies how NGS provides clarity in culture-negative PJI cases and enables precision medicine, even in complex revision cases.
Case Spotlight #2: NGS Reveals Joint Infection Masked as Neurodegenerative Disease
 At AdventHealth Waterman, a 49-year-old HIV-positive man with a history of neurosyphilis presented with knee pain and swelling. Imaging initially suggested Charcot arthropathy rather than infection.
At AdventHealth Waterman, a 49-year-old HIV-positive man with a history of neurosyphilis presented with knee pain and swelling. Imaging initially suggested Charcot arthropathy rather than infection.
Though the patient presented with tabetic joint disease, synovial fluid revealed >40,000 WBCs while cultures remained negative.
A sample was sent to MicroGenDX, and our qPCR + NGS assay reported Mycobacterium tuberculosis in 48 hours (now next-day upon receipt).
Dr. Corey Hall Henderson, Infectious Disease specialist, immediately ordered the patient to be isolated and RIPE therapy to begin. The patient’s recovery followed while cultures were still growing.
While the infection masqueraded as a degenerative neuropathic joint disease, molecular testing delivered a diagnostic homerun to uncover the true culprit – restoring a man’s ability to walk.
From Uncertainty to Precision: How NGS Transforms Clinical Decision-Making
 Moving to NGS represents a shift to targeted antimicrobial and surgical strategies.
Moving to NGS represents a shift to targeted antimicrobial and surgical strategies.
When cultures fail, clinicians default to empiric therapy without pathogen confirmation. This uncertainty complicates antimicrobial selection and the decision between single-stage and two-stage revision.
NGS bridges this diagnostic gap by delivering microbe-level insight and infection-level drug resistance screening with the qPCR test for a more complete microbial profile. With a data-driven microbial profile of the infection, NGS supports:
- More effective targeted therapy, particularly against low-virulence or biofilm-associated organisms.
- Reduction in unnecessary broad-spectrum antimicrobial exposure, aligning with antimicrobial stewardship goals.
- Increased confidence in surgical planning, particularly in determining the feasibility of single-stage revision.
Moreover, this actionable clarity enables faster initiation of targeted therapy, shortens hospital stays, and minimizes costs and complications from ineffective treatments.
Moving Toward Swift, Data-Driven Care
 Culture-negative PJIs persistently challenge orthopedic teams, delaying effective treatment and complicating revision plans. As demonstrated in recent clinical studies and patient cases, NGS resolves this diagnostic gap by uncovering pathogens in culture-negative PJIs, especially polymicrobial infections that culture routinely misses. This shift provides swifter diagnostic insight than traditional culture, enabling faster, more personalized care strategies.
Culture-negative PJIs persistently challenge orthopedic teams, delaying effective treatment and complicating revision plans. As demonstrated in recent clinical studies and patient cases, NGS resolves this diagnostic gap by uncovering pathogens in culture-negative PJIs, especially polymicrobial infections that culture routinely misses. This shift provides swifter diagnostic insight than traditional culture, enabling faster, more personalized care strategies.
To support that speed, MicroGenDX offers a free mobile app that alerts physicians the instant patient results are ready and provides access to full reports.
Real-world cases demonstrate how NGS can reduce treatment burden and support more confident surgical and discharge decisions by care teams.
Looking ahead, NGS is expected to have an expanded role in orthopedic infection management, including:
- Greater integration into clinical guidelines, as evidence from clinical studies continues to mount.
- Increasing payer recognition and more streamlined reimbursement, particularly for culture-negative and recurrent infection cases where diagnostic clarity offers high clinical value and reduces cost.
With rising arthroplasty volumes and increasingly complex revision procedures, orthopedic teams will call upon tools that provide early and accurate pathogen identification to enable tailored therapy.
NGS is well-positioned to drive this shift in orthopedics and beyond.
Interested in bringing OrthoKEY to your practice, clinic, or hospital?
📞 Call Customer Service: 1-855-208-0019
📧 Email: [email protected]
🌐 Learn more about or order OrthoKEY PJI OR and OrthoKEY PJI Clinic.